Conductors, Insulators and Semiconductors
CONDUCTORS
Any material which allows current to flow is called a conductor. Metals are a good conductor of electricity. An electrical conductor is a substance in which electrical charge carriers, usually electrons, move easily from atom to atom with the application of voltage. Conductivity, in general, is the capacity to transmit something, such as electricity or heat. Copper, steel, gold, aluminum, and brass are good conductors of electricity.
They are mostly metals like Cu, Al, and other alloys. The choice of the conductor can be taken into account by considering various factors like tensile strength conductivity, local conditions, and cost.
Applications of Conductors
Conductors are quite useful in many ways. They are used in many real-life applications like:
- Mercury is a common ingredient in thermometers to check the temperature of the body.
- Aluminum is commonly used for making foils to store food and for the fast storage of heat in the production of fry pans.
- Iron is common in vehicle engine manufacturing to conduct heat. An iron plate is made up of steel to absorb heat quickly.
- In car radiators conductors are used to eliminate heat away from the engine.
INSULATORS
An insulator is a material that does not transmit electrical current or an insulator is a material with such low conductivity that the current flow through it is negligible. Insulating materials include paper, plastic, rubber, glass and air.
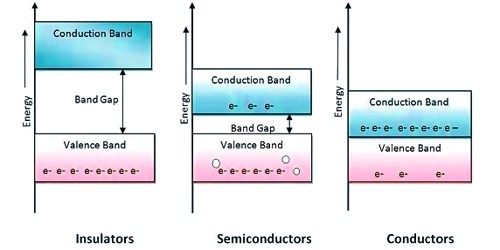
In insulators, the valence band is fully occupied with electrons due to the covalent bonds. The electrons cannot move because they're "locked up" between the atoms. To achieve conductivity, electrons from the valence band must move into the conduction band. Electrical wires are coated with a layer of insulation.
The most common difference between the two is that while conductors allow free flow of electrons from one atom to another, insulators restrict the free flow of electrons. Conductors allow electrical energy to pass through them, whereas insulators do not allow electrical energy to pass through them.
Applications of Insulators
Being resistive to the flow of electrons, insulators find uses in a variety of forms around the world. Some of the common uses include:
- Thermal insulators do not allow heat to move from one place to another. We use them in making thermoplastic bottles, in fireproofing ceilings and walls.
- Sound insulators help in controlling noise levels by preventing the sound from being transmitted.
- Electrical insulators hinder the flow of electrons or passage of current through them. We use them extensively in circuit boards, high-voltage systems and in coating of electrical wires and cables.
- A semiconductor material has an electrical conductivity value falling between that of a conductor, such as metallic copper, and an insulator, such as glass.
- By adding a small percentage of another element to the base material i.e. silicon or germanium and by controlling the amount of impurities added to the semiconductor material, it is possible to control its conductivity.
- Various impurities called donors or acceptors can be added to the semiconductor material to produce free electrons or holes respectively. This process is known as doping of semiconductors.
- Semiconductors have few free electrons as their atoms are closely grouped in a crystal lattice and are only able to flow under special conditions.
List of Common Semiconductor Devices
The list of common semiconductor devices mainly includes two terminals, three terminals, and four-terminal devices.
Types of Semiconductors
Semiconductors can be classified as:
- Intrinsic Semiconductor
- Extrinsic Semiconductor
INTRINSIC SEMICONDUCTOR
An intrinsic(pure) semiconductor, also called an undoped semiconductor, is a pure semiconductor without any noticeable presence of dopant material. Therefore, the number of charge carriers is determined by the properties of the material itself rather than by the sum of impurities. It is made up of only a single type of element.
Germanium (Ge) and Silicon (Si) are the most common type of intrinsic semiconductor elements. They have four valence electrons (tetravalent). They are bound to the atom by a covalent bond at absolute zero temperature.
When the temperature rises, due to collision, few electrons are unbounded and become free to move through the lattice, thus creating an absence in its original position (hole). These free electrons and holes contribute towards the conduction of electricity in the semiconductor. The negative and positive charge carriers are equal in number.
The thermal energy can ionize a few atoms in the lattice, and hence their conductivity is less.
In intrinsic semiconductors, current flows due to the motion of free electrons as well as holes. The total current is the sum of the electron current Ie due to thermally generated electrons and the hole current Ih
Total Current (I) = Ie + Ih
For an intrinsic semiconductor, at finite temperature, the probability of electrons to exist in conduction band decreases exponentially with increasing bandgap (Eg)
n = n0e-Eg/2.Kb.T
Where,
- Eg = Energy bandgap
- Kb = Boltzmann’s constants
EXTRINSIC SEMICONDUCTOR
The conductivity of semiconductors can be greatly improved by introducing a small number of suitable replacement atoms called impurities. The process of adding impurity atoms to the pure semiconductor is called doping. Usually, only 1 atom in 107 is replaced by a dopant atom in the doped semiconductor. An extrinsic semiconductor can be further classified into:
- N-type Semiconductor
- P-type Semiconductor
Classification of Extrinsic Semiconductor
N-TYPE SEMICONDUCTOR
- Mainly due to electrons
- Entirely neutral
- I = Ih and nh >> ne
- Majority – Electrons and Minority – Holes
When a pure semiconductor (Silicon or Germanium) is doped by pentavalent impurity (P, As, Sb, Bi) then, four electrons out of five valence electrons bond with the four electrons of Ge or Si.The fifth electron of the dopant is set free. Thus, the impurity atom donates a free electron for conduction in the lattice and is called “Donor“.
Since the number of free electrons increases by the addition of an impurity, the negative charge carriers increase. Hence it is called an n-type semiconductor.
Crystal is neutral, but the donor atom becomes an immobile positive ion. As conduction is due to many free electrons, the electrons in the n-type semiconductor are the MAJORITY CARRIERS and holes are the MINORITY CARRIERS.
P-TYPE SEMICONDUCTOR
- Mainly due to holes
- Entirely neutral
- I = Ih and nh >> ne
- Majority – Holes and Minority – Electrons
When a pure semiconductor is doped with a trivalent impurity (B, Al, In, Ga ), three valence electrons of the impurity atom bonds with three of the four valence electrons of the semiconductor.This leaves an absence of an electron(hole) in the impurity. These impurity atoms which are ready to accept bonded electrons are called “Acceptors“.
With the increase in the number of impurities, holes (the positive charge carriers) are increased. Hence, it is called a p-type semiconductor.
Crystal is neutral, but the acceptors become an immobile negative ion. As conduction is due to many holes, the holes in the p-type semiconductor are MAJORITY CARRIERS and electrons are MINORITY CARRIERS.
APPLICATIONS OF SEMICONDUCTOR
- Temperature sensors are made with semiconductor devices.
- Semiconductors are used in 3D printing machines
- Used in microchips and self-driving cars
- Used in calculators, solar plates, computers, and other electronic devices.
- MOSFETs and Transistors used as a switch in electrical circuits are manufactured using semiconductors.